01 DESIGN BRIEF -
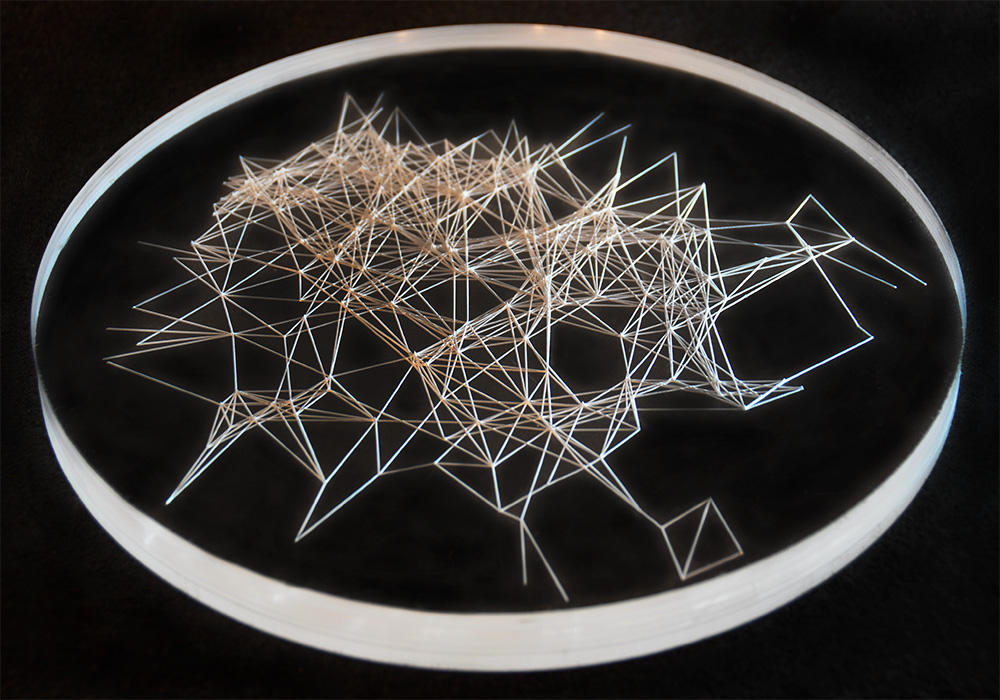
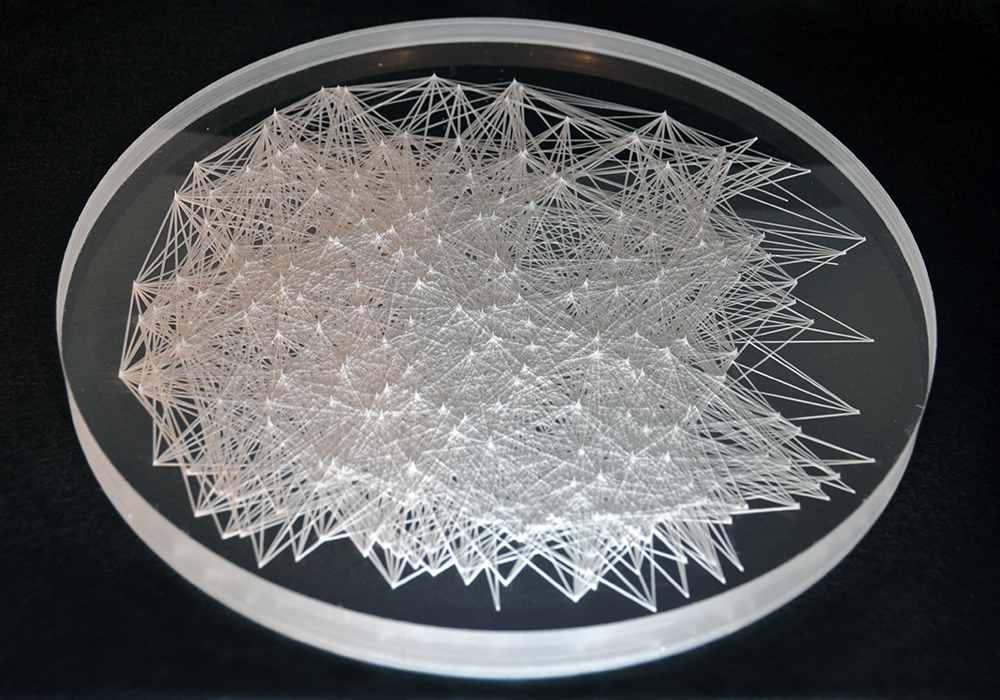
Carbon dioxide becomes more soluble in water as the temperature decreases. This series of laser cut disks demonstrates the change in carbon dioxide’s concentration as water freezes. By analyzing the chemical bonding in the carbon dioxide and water molecules, points of intersection are used to indicate the molecules while the lines represent the chemical bondings between the elements.
02 DESIGN PROCESS -
Due to the nature of bonding of carbon dioxide in water molecules, carbon dioxide become less soluble in water as temperature increases. Below is a graph showing solubility of carbon dioxide in water under different temperatures. As we can see in the graph, solubility of carbon dioxide in water decreases inversely exponentially as temperature of water increases.
Graph from Chemistry for Life website
03 FINAL DELIVERABLES -
1. Disk Close-ups
2. Disks Full View
3. Disks Installation View
04 INTERACTIVE INSTALLATION -
This project examines the relationship between human activities and carbon dioxide production. When more movement is captured, more particles are produced. This indicates more carbon dioxide molecules are released into the air during periods of increased velocity. This phenomenon provides a way to reflect on how our daily activities directly influence the global environment. With more human activities, more carbon dioxide is produced together in combination with other pollutants to inflict harm on our mother earth.